Consumer Diary: Drug Discounts, Weight Loss Scam

Audio By Carbonatix

You can get big discounts for various drugs that are not covered by insurance. You just point your smart phone camera at the QR code, and you’ll be directed to websites providing discounts. Photo credit: Harlan Levy
Consumer columnist and West Hartford resident Harlan Levy has more than 20 years of experience writing stories about everyday experiences that anyone could encounter.

Harlan Levy. Courtesy photo
By Harlan Levy
Here’s a little consumer story: I’ve bought a prescription-only super-effective toothpaste, Prevident, that my dentist highly recommended, twice in the last year, each time paying its $47 price. My ConnectiCare insurance does not cover it, unfortunately. Twice I got it at the drive-up window at Walgreens.
Last week I bought it inside the store and asked the clerk if there were any discounts for this high-priced product. He checked and found one, cutting $22 off the cost! When I paid the $25 he pointed to a sign at the register which gives customers discounts for various prescriptions. All you have to do is point your smart phone camera at the square QR code, which directs you to websites offering discounts. Buying Prevident from the drive-up lane did not give me the chance to get the discount.
NOTE: CVS also offers such discounts with its SingleCare prescription discount card to save up to 80% on prescription drugs at CVS pharmacies.
So now you know – if you don’t already.
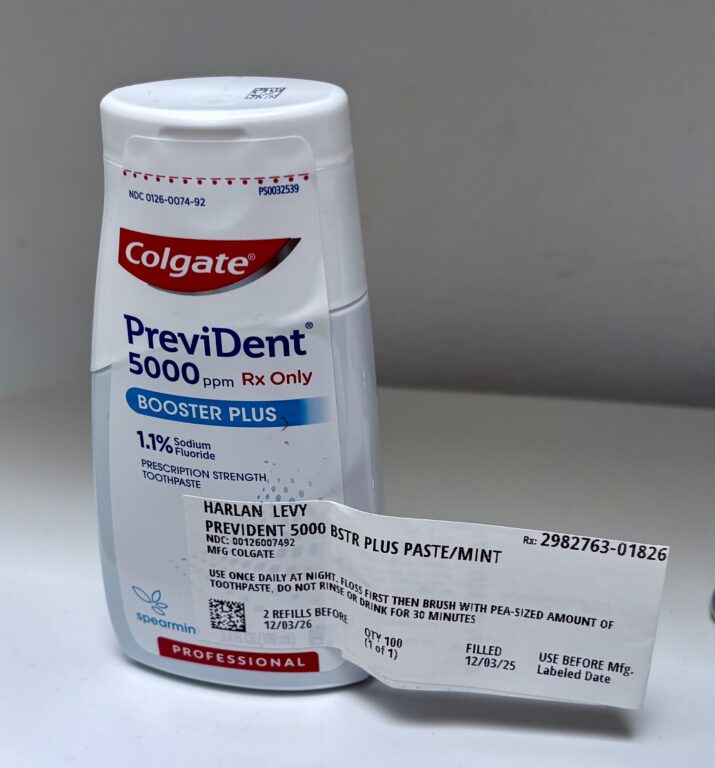
PreViDent is my extra effective prescription-only tooth paste, costing $47. My ConnectiCare insurance doesn’t cover it. Photo credit: Harlan Levy
Scam refund
The Federal Trade Commission last week said the following: It is sending payments totaling more than $27.6 million to consumers – including those in Connecticut – who were enrolled, without their knowledge, in plans where they were shipped and charged repeatedly for products marketed to promote weight loss, clear skin, and other healthcare benefits.
Defendants Legion Media LLC, KP Commerce LLC, Pinnacle Payments LLC, Sloan Health Products LLC, and their principals, operated two types of unauthorized billing scams. In the first, the FTC alleged that the defendants defrauded consumers who bought CBD and Keto-related products by charging them more than the advertised price and enrolling them in continuity plans without their consent in which they are charged for products they never intended or agreed to buy. Several defendants participated in a second scheme where consumers paid a small shipping fee for a supposedly free “gift.” However, after consumers used their credit and debit cards to pay the fee, they incurred recurring unauthorized charges on their cards.
The FTC is sending checks and PayPal payments to 1,215,337 affected consumers between now and Dec. 18, 2025. Most consumers will receive a check in the mail. Check recipients should cash their checks within 90 days. PayPal recipients should redeem their PayPal payments within 30 days.
The agency never requires people to pay money or provide account information to get a refund.
The FTC also reported that in 2024 FTC actions led to more than $339 million in refunds to consumers across the country, including $23.11 million in refunds sent to 151,577 Connecticut victims.
Sunscreens
Summer feels far away, but here’s some good news: On Dec. 11, the U.S. Food and Drug Administration announced that it has proposed adding bemotrizinol as an active ingredient in over-the-counter sunscreens, the kind of effective sun protection used abroad for decades. The new ingredient gives adults and children 6 months and older access to a much-needed non-mineral option with stronger UVA protection.
Bemotrizinol provides strong broad-spectrum protection, safeguarding consumers against both ultraviolet A and B, or UVA and UVB, rays. UVA fradiation can penetrate the skin more deeply than UVB rays and is associated with skin aging and cancer, including melanoma.
The ingredient also shows very low absorption through the skin and rarely causes irritation, the agency says.
When the FDA finalizes its plan, bemotrizinol would become the first new active sunscreen ingredient allowed on the U.S. market in decades. The FDA published its review of the ingredient in the Federal Register on Dec. 12 and will take public comment on it for 45 days. So a final decision could be out in the spring – just in time for summer.
NOTE: If you have a consumer problem, contact me at [email protected] (“Consumer” in subject line), and, with the power of the press, maybe I can help.
Like what you see here? Click here to subscribe to We-Ha’s newsletter so you’ll always be in the know about what’s happening in West Hartford! Click the blue button below to become a supporter of We-Ha.com and our efforts to continue producing quality journalism.



